Abstract
Background Comprehensive genomic analysis has identified subgroups of diffuse large B cell and high grade B cell lymphoma (DLBCL/HGBL) patients (pts) who experience inferior survival outcomes following receipt of first-line treatment (1Ltx) with immunochemotherapy (PMID: 29713087, 29641966). However, this type of analysis has not been performed for pts with R/R DLBCL/HGBL treated with curative-intent IC in the 2L, although descriptive analyses of mutations (mut) present at the time of first R/R DLBCL/HGBL have been reported (PMID: 26647218, ICML 2017 #294). Here we report mut identified by clinical laboratory mutation analysis (CLMA) in tumor biopsies (bx) obtained at the time of first R/R DLBCL/HGBL in patients subsequently receiving 2Ltx with curative-intent IC with analysis of response and survival outcomes.
Methods Pts diagnosed with first R/R DLBCL/HGBL after receipt of 1Ltx who underwent tumor bx at that time with diagnostic CLMA performed at the University of Pennsylvania from 2015-21 with one of three lymphoma sequencing panels who were treated with RICE or RDHAP/C as 2Ltx were retrospectively analyzed. Immunohistochemical staining (IHC) for MYC and BCL2 expression as well as fluorescence in situ hybridization (FISH) for MYC, BCL2 and BCL6 rearrangements were performed as per institutional standard. 2Ltx was given at the discretion of the treating physician. Disease free survival (DFS) was defined as the interval between diagnosis of first R/R DLBCL/HGBL to next occurrence of R/R DLBCL/HGBL or last follow-up in remission. Overall survival (OS) was defined as the interval between diagnosis and time of death or last follow-up while alive. Data were censored on 7/1/22.
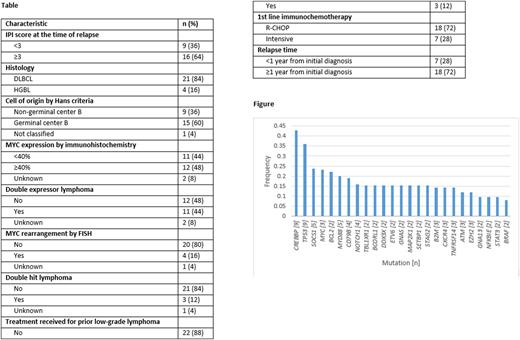
Results CLMA was performed on 28 pt tumor bx with 25 successful assays (89% success rate) and a median result turnaround time (TAT) of 17 days. Baseline clinicopathologic and tx characteristics are listed in the Table and mutations detected by frequency with n≥1 in the Figure. 2Ltx was RICE in 18 (72%) and R-DHAP/C in 7 (28%) pts. With a median follow-up of 32.4 months from the time of first R/R disease, the Kaplan Meier estimate of 2 year (y) DFS was 12% (95% confidence interval [CI] 2-31%) and 2y OS 59% (95% CI 33-78%). Response to 2Ltx was achieved by 18 pts (72%). Additional analysis was performed to determine factors predictive of response to 2Ltx and 2y DFS using the characteristics in Table 1, 2Ltx received as well as mutation detected in ≥5 cases (CREBBP, TP53, SOCS1 and MYD88). Those predictive of response to 2Ltx with P<0.10 on univariate logistic regression were receipt of R-DHAP/DHAC vs RICE (odds ratio [OR] 0.15, 95% CI 0.02-1.0, P=0.06) and CREBBP mutation vs not (OR 0.07, 95% CI 0.01-0.83, P=0.04). Those predictive of disease relapse by 2y with P<0.10 on univariate Cox regression were HGBL vs DLBCL histology (hazard ratio [HR] 4.8, 95% CI 1.4-16.4, P=0.01), receipt of R-DHAP/DHAC vs RICE (HR 4.7, 95% CI 1.6-13.8, P=0.005), GCB vs non-GCB cell of origin (HR 1.9, 95% CI 0.94-4.0, P=0.07), MYC rearrangement vs not (HR 4.5, 95% CI 1.3-15.4, P=0.02) and double hit lymphoma vs not (HR 3.8, 95% CI 1.0-14.5, P=0.046). Meaningful multivariate analyses for response to 2Ltx or disease relapse at 2y could not be carried out due to small sample size.
Conclusion In this cohort of pts with DLBCL/HGBL whose tumor bx obtained at the time of first R/R disease underwent CLMA and were treated with RICE or RDHAP/C as 2Ltx, few patients achieved long-term DFS in spite of a high rate of chemosensitivity to 2Ltx. Mut in CREBBP predicted for a lower response rate to 2Ltx but not disease progression at 2y, and no other mut analyzed predicted for either outcome. Given the low rate of 2y DFS as well as a shift in practice to the use of chimeric antigen receptor-modified T cells (CART) as 2Ltx for pts with early R/R DLBCL/HGBL, these data do not support further investigation of CLMA performed on a larger sample of tumor bx from all pts at the time of first R/R disease who are candidates for CART. However, performance of CLMA could further explored in the subset of DLBCL/HGBL pts with late first R/R disease for whom the standard of care 2Ltx remains curative-intent IC, as the presence of absence of mut predicting for disease response/DFS in this pt population may inform tx decisions given the availability of non-cytotoxic agents as 2L tx as well as CART at the time of subsequent R/R disease.
Disclosures
Schuster:Regeneron: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; Legend Biotech: Consultancy; Loxo: Consultancy; Morphosys: Consultancy; MustangBio: Consultancy; Nordic: Consultancy; Nanovector: Consultancy; Novartis: Consultancy, Research Funding; Adaptive Biotechnologies: Research Funding; DTRM: Research Funding; Juno Therapeutics: Research Funding; Merck: Research Funding; Abbvie: Research Funding; TG Therapeutics: Research Funding; Pharmacyclics: Research Funding; Incyte: Consultancy, Research Funding; Genetech/Roche: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; BiGene: Consultancy; Acerta: Consultancy. Dwivedy Nasta:FortySeven/Gilead: Research Funding; Pharmacyclics: Research Funding; Roche: Research Funding; Rafael: Research Funding. Gerson:Loxo Oncology: Research Funding; Genentech: Consultancy; Abbvie: Consultancy. Barta:Janssen: Other: Independent Data Monitoring Committee member; Daiichi Sankyo: Consultancy; Kyowa Kirin: Consultancy, Honoraria; Acrotech: Honoraria; Seagen: Honoraria; Affimed: Consultancy. Svoboda:TG: Research Funding; SEAGEN: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Merck: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Consultancy; BMS: Consultancy, Research Funding; Atara: Consultancy; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADCT: Consultancy. Chong:Novartis: Consultancy; Beigene: Consultancy; Juno/BMS: Consultancy; Tessa: Consultancy; KITE: Consultancy. Lim:EUSA Pharma: Honoraria. Landsburg:Morphosys: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Epizyme: Membership on an entity's Board of Directors or advisory committees; Curis, Inc: Research Funding; Calithera: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Triphase: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal